 |
|||||||||||||||||
|
|||||||||||||||||
|
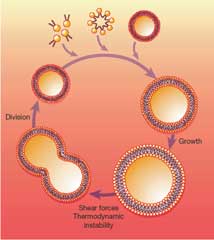
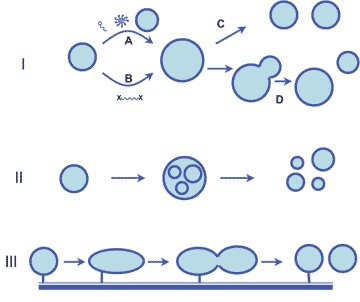
Vesicle Replication
Primitive cells, lacking the complex biomachinery present in modern cells, would have had to rely on the self-organizing properties of their components and on interactions with their environment to achieve basic cellular functions such as growth and division.
Many bilayer-membrane vesicles can exhibit complex morphological changes such as growth, fusion, fission, budding, internal vesicle assembly and vesicle-surface interactions. The rich dynamic properties of these vesicles provide interesting models of how primitive cellular replication might have occurred in response to purely physical and chemical forces. We have engineered a system of vesicle replication with discrete growth and division steps. When micelles comprised of myristoleate were added to myristoleic acid/myristoleate vesicles, we observe vesicle growth as well as new vesicle formation. By controlling the rate of addition of micelles to eliminate high concentrations that favor their self-association, vesicle growth can be promoted at the expense of new vesicle formation. Osmotic swelling of vesicles produces a high energy state as compared to relaxed (isotonic) vesicles, and membrane components may be transferred from relaxed, low energy vesicles to swollen, high energy vesicles, minimizing the overall energy of the mixture and resulting in the growth of the swollen vesicles. In one example of this phenomenon, RNA encapsulated in fatty acid vesicles is used to exert an osmotic pressure on the vesicle membrane that drives the uptake of additional membrane components, leading to membrane growth at the expense of relaxed vesicles, which shrink. This observation supports a model in which more efficient RNA replication could result to more rapid vesicle growth, leading to the emergence of Darwinian evolution in primitive cells. We have also shown that mineral surfaces can accelerate the assembly of vesicles from fatty acid micelles. A dispersion of montmorillonite clay in buffer accelerates the assembly of vesicles from fatty acid micelles. When the vesicles form, some of the clay becomes encapsulated in large vesicles, which in turn become packed full of smaller vesicles. Division of vesicles can be achieved by extruding the grown vesicles through small pores, so that the extruded vesicles have the same diameter as the initial population before vesicle growth. By monitoring a fluorescent dye encapsulated within the initial vesicles, we have shown that the division process occurs with very little loss or dilution of the vesicle contents over multiple cycles of growth and extrusion. This simple system serves as a proof of principle that primitive cellular replication could have occurred through purely physico-chemical forces, such as fluid flowing through porous rocks near hydrothermal vents. Click here for a video of vesicle extrusion [.avi format, approx. 7MB] Left-click to play the video, or right-click and choose "Save Target As" to download a copy to your PC. Developing Vesicles to Support Ribozyme Function Our efforts to build a simple cell comprised of a membrane and a nucleic acid-based metabolism begin with establishing conditions that allow ribozymes to function within vesicles. Using a mixture of myristoleic acid and its glycerol monoester we have constructed vesicles that were Mg2+-tolerant. Membranes made from these simple amphiphiles can form vesicles that are stable enough to retain This combination of stability and dynamics is critical for building model protocells in the laboratory and may have been important for early cellular evolution. |
|||||||||||||||||
|
|
|||||||||||||||||
| Copyright © 2006-2012 The Massachusetts General Hospital | |||

 encapsulated RNAs in the presence of divalent cations, yet dynamic enough to grow spontaneously and allow the passage of Mg2+ and mononucleotides without specific macromolecular transporters.
encapsulated RNAs in the presence of divalent cations, yet dynamic enough to grow spontaneously and allow the passage of Mg2+ and mononucleotides without specific macromolecular transporters.