|
|||||||||||||||||
|
|||||||||||||||||
|
Drug Discovery
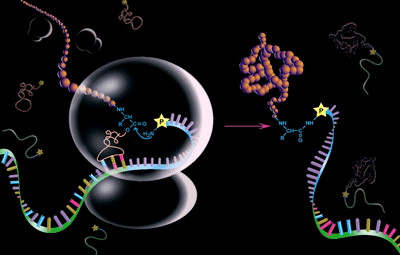
The key tool for the selection of protein sequences with novel binding or catalytic activity is mRNA Display (FIGURE and description). The mRNA-display approach uses the peptidyl-accepting antibiotic puromycin, chemically appended to the 3’ of the mRNA, to provide a covalent link between the nascent peptide and its coding mRNA prior to release from the ribosome. This physical linkage between the ribosomally synthesized protein (phenotype) and the mRNA that codes for its synthesis (genotype) allows for the simultaneous isolation of the desired functional protein and the encoding mRNA. While mRNA display has been used extensively by our laboratory and others in the selection of proteins with novel sequences comprised of the 20 naturally-occurring amino acids, we have greatly expanded its utility by establishing conditions for the incorporation non-natural amino acids. Using a fully-defined in vitro translation system comprised of highly purified E. coli ribosomes, tRNAs, and recombinant translation factors and amino acid tRNA synthetases, and omitting naturally-occurring amino acids, we have been able to charge individual tRNAs with a wide variety of non-natural amino acids. These charged tRNAs can participate in mRNA-directed, ribosome-mediated peptide-bond synthesis. This system is fully compatible with the synthesis of puromycin-mediated molecular fusions between the unnatural peptide and its mRNA for the in vitro selection of drug-like molecules by mRNA-display. Non-natural amino acids under evaluation include D-amino acids, beta-amino acids, N-methyl amino acids, and amino acids with modified, novel, or chemically-reactive side chains. We predict that peptides synthesized by this approach will greatly expand the chemical diversity of peptide libraries and further allow the synthesis of peptides with desirable “drug-like” properties such as protease resistance, chemical stability, cyclic structures, and enhanced binding capabilities with in vivo targets. |
|||||||||||||||||
|
|
|||||||||||||||||
| Copyright © 2006-2012 The Massachusetts General Hospital | |||